
Brf3 Polar Or Nonpolar
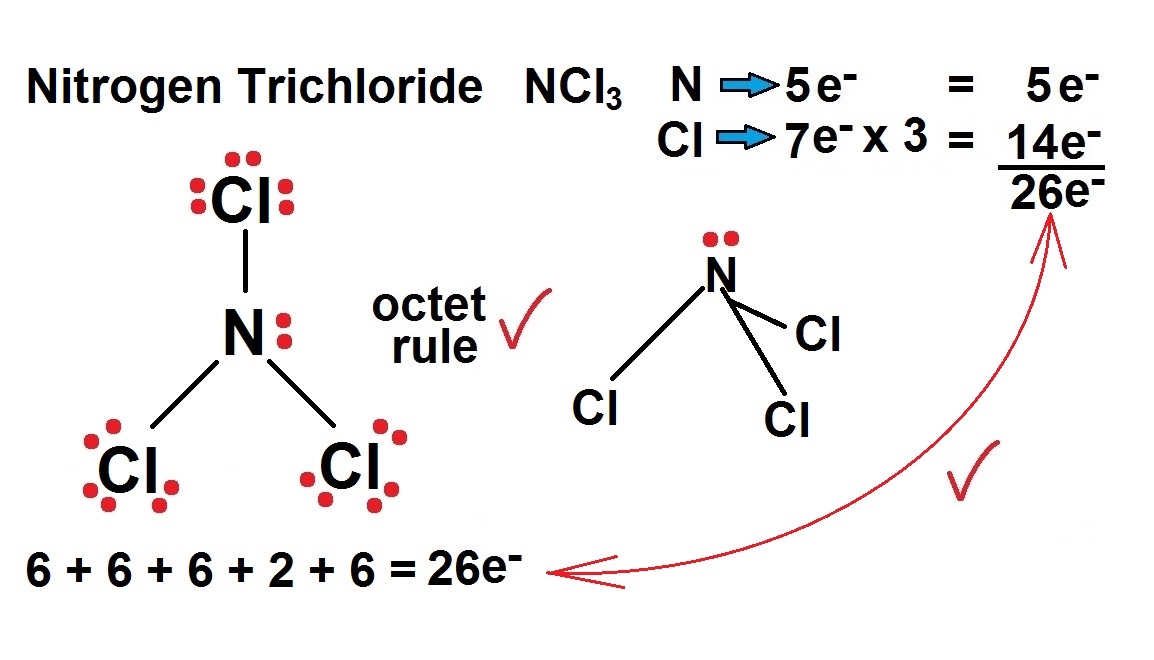
NCl3 has a Lewis structure that is similar to NF3. One nitrogen atom is in the middle, with three chlorine atoms equally distributed around it. The core atom of the NCl3 Lewis dot structure has one lone pair, whereas each chlorine atom has three. Follow these steps to make the Lewis dot structure of NCl3.

Is NCl3 Polar or Nonpolar? Techiescientist
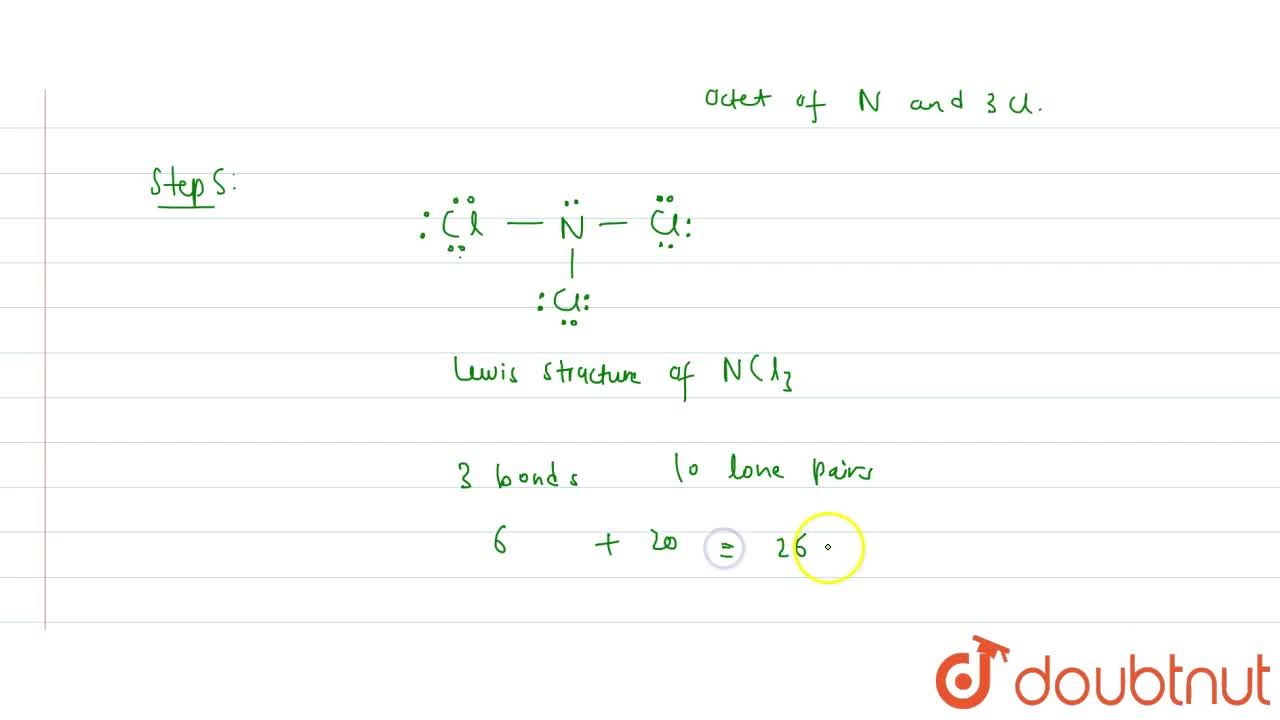
Lewis structure of NCl3 contains three single bonds between the Nitrogen (N) atom and each Chlorine (Cl) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Chlorine atoms (Cl). The Nitrogen atom has 1 lone pair and all the three Chlorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.
PPLATO FLAP PHYS 8.4 The periodic table and chemical bonding
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons constitutes a single bond. Each.

sobre " Ncl3 " e polar ou apolar? existe nessa molécula 3 ligações covalentes normais? a
Lewis Structure of NCl3, Nitrogen Trichloride chemistNATE 245K subscribers 12K views 2 years ago Lewis Structures Nitrogen and chlorine are both non-metals, so they SHARE electrons to.

NCl3 Lewis StructureLewis Structure of NCl3 (Nitrogen Trifluoride)Draw Lewis Structure for
NCl3 lewis structure contains three N-Cl bonds, nitrogen in the center position whereas all three chlorine atoms are at the terminal position. There is only one lone pair present on the central atom in the NCl3 lewis structure. Let's see how to draw its Lewis structure in a simple way-

How to draw NCl3 Lewis Structure? 2
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl 3. This yellow, oily, pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia -derivatives and chlorine (for example, in swimming pools ).

So far, we’ve used 26 of the NCl3 Lewis structure’s total 26 outermost valence shell electrons
In the NCl 3 Lewis structure, there are three single bonds around the nitrogen atom, with three chlorine atoms attached to it. Each chlorine atom has three lone pairs, and the nitrogen atom has one lone pair. NCl3 Lewis Structure - How to Draw the Dot Structure for NCl3 Watch on Contents Steps #1 Draw a rough skeleton structure
23+ Ncl3 Lewis Structure Molecular Geometry Gif GrAffiTi
Lewis Structure of Nitrogen Trichloride (NCl3) The Lewis structure is the foremost step to begin studying the physical and chemical properties of any molecule. For nitrogen trichloride, it is essential to study the Lewis structures of the participating atoms before drawing the one for the molecule.

NCl3 Lewis Structure How to Draw the Dot Structure for NCl3 YouTube
A step-by-step explanation of how to draw the NCl3 Lewis Dot Structure (Nitrogen trichloride).For the NCl3 structure use the periodic table to find the total.
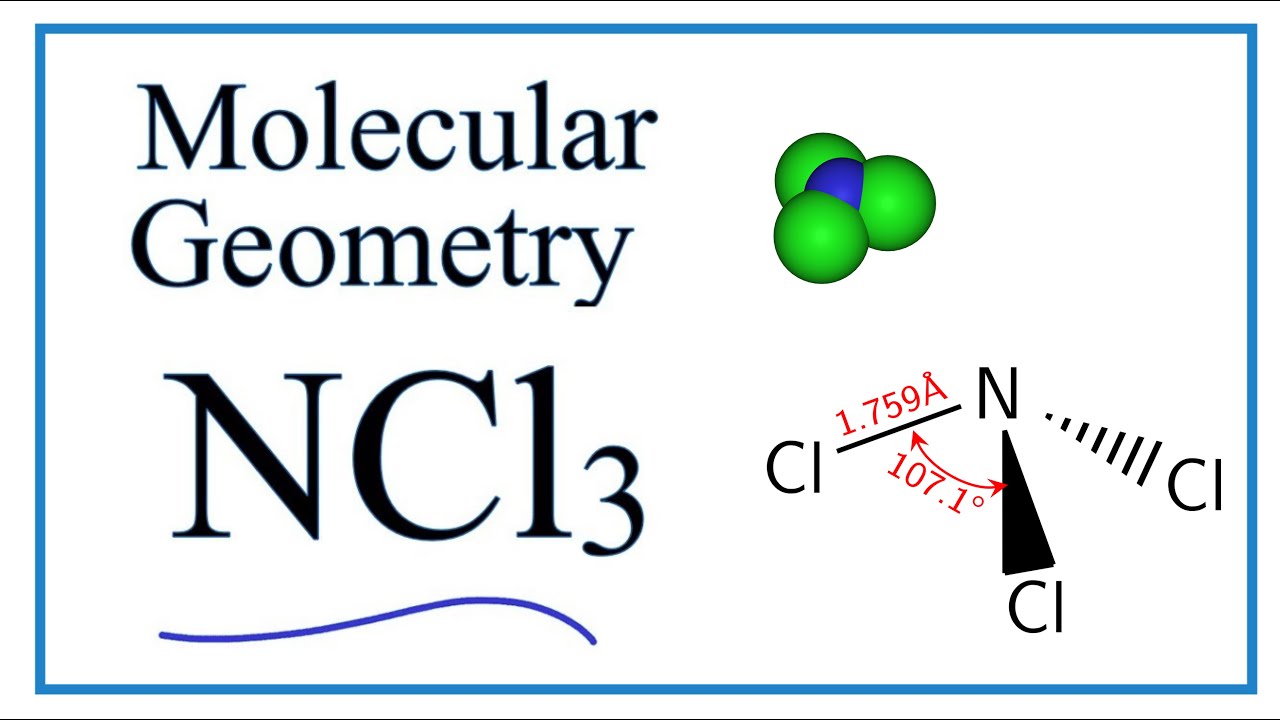
NCl3 Molecular Geometry / Shape and Bond Angles YouTube
The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell is called.
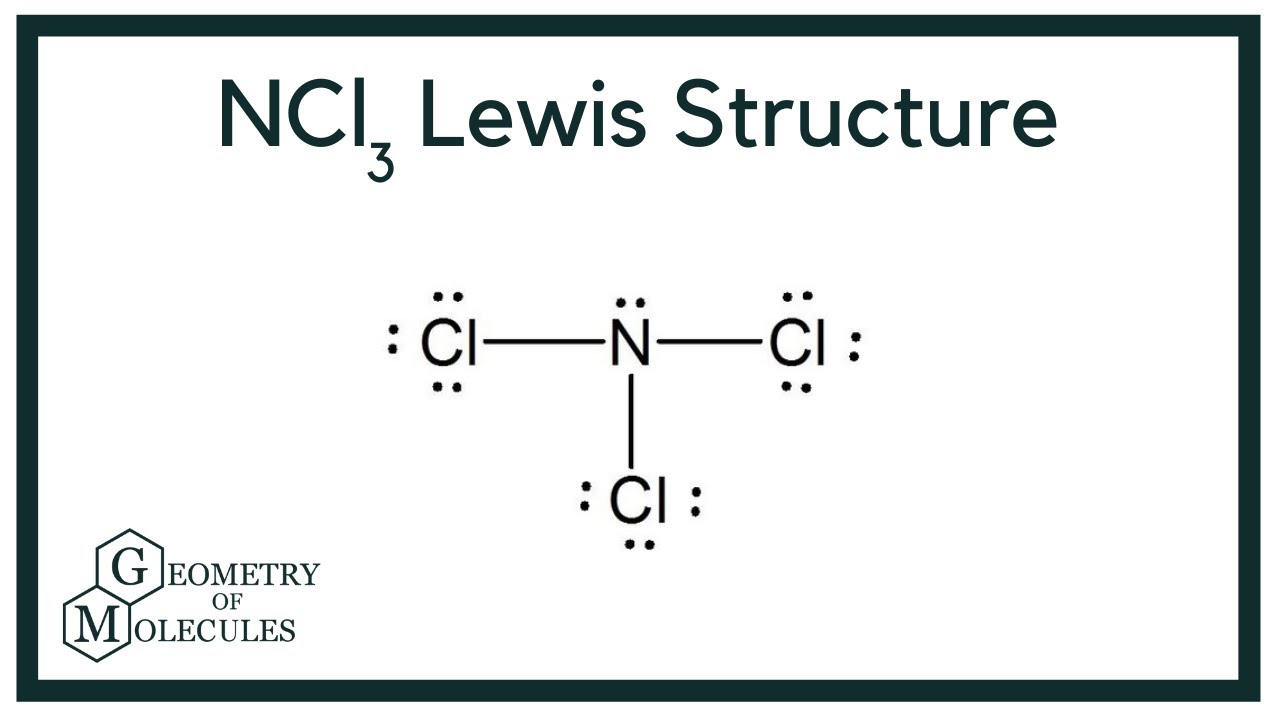
NCl3 Lewis Structure (Nitrogen Trichloride) YouTube
A quick explanation of the molecular geometry of NCl3 (Nitrogen trichloride) including a description of the NCl3 bond angles..more.more Molecular Geometry: Rules, Examples, and Practice.

NCl3 Lewis Structure, Geometry, Hybridization, and Polarity Techiescientist
Determine the formal charge for each atom in \(\ce{NCl3}\). Answer. N: 0; all three Cl atoms: 0.. In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure.
Lewis Structure Ncl3
Step-1: NCl3 Lewis dot Structure by counting valence electrons on the Nitrogen atom Step-2: Lewis Structure of NCl3 for counting valence electrons around the terminal chlorine atoms Step-3: Lewis dot Structure for NCl3 generated from step-1 and step-2 How to calculate the formal charge on Nitrogen and Chlorine atoms in NCl3 Lewis Structure?
lewis structure for nitrogen trifluoride
Lewis structure of NCl 3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Also, there are no charges on atoms in NCl 3. Steps of drawing the lewis structure of NCl 3 are explained in detail in this tutorial. Lewis structure of NCl 3

Lewis Structure of NCl3, Nitrogen Trichloride YouTube
Lewis structure of nitrogen trichloride (NCl3) contains three sigma bonds and one lone pair around nitrogen atom. Therefore, there are total of four electrons regions. So, hybridization of phosphorus atom is sp3. Because there are four electrons regions, geometry is tetrahedral and shape is trigonal pyramidal.
no2 bond order
How to Draw a Lewis Structure for NCl3? Lewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Subscribe: https:.